:focus(728x321:729x322))


With global battery markets expected to reach $86.6 billion by 2018, environmental concerns around their production and disposal are grave. Scientists at UEA are helping to understand how clean energy may be generated with help from a surprising source - bacteria.
From smartphones to laptops the use of portable technology is widespread – and synonymous with its use are the batteries that power them. With a booming global consumer electronics industry (mobile phone subscriptions alone now reach almost 7 billion) battery consumption is only going to escalate.
Batteries work by turning chemical energy into electrical energy, providing an easy, portable source of power but their production and disposal can lead to serious environmental damage, from the impact of mining minerals to the pollution caused by their incorrect disposal.
Biochemical scientists at UEA are looking to understand how, in the right environment, bacteria can actually generate electricity, something that could provide alternative solutions to powering the world’s technology.
Unlike humans, many micro-organisms can survive without oxygen. To do this bacteria including species of Shewanella provide a flow of electricity as a side product of respiration. These bacteria internalise simple carbon-based molecules from their environment, combust them to harness energy and this releases a flow of electrons back out of the cell across the bacterial outer membrane.
In sediments and soils the electricity is delivered to mineral oxide particles containing Fe(III) and Mn(IV) earning Shewanella the moniker of rock-breathing bacteria. In microbial fuel cells the electricity is delivered to graphite electrodes and enters into an electrical circuit.
Professor Julea Butt and Dr Tom Clarke from UEA’s School of Chemistry and School of Biological Sciences are uncovering how electrons are moved from the inside to the outside of bacteria. Their research teams study a group of proteins – known as multiheme cytochromes – that underpin this process in Shewanella oneidensis.

Heme cofactors support electron transfer because they contain iron that can exist in reduced, Fe2+, and oxidised, Fe3+, states. Multiheme cytochromes position several heme factors in close proximity in order to allow electrons to move from one side of the protein to another. When these cytochromes span the bacterial outer membrane electrons move from the inside to the outside of the cell.
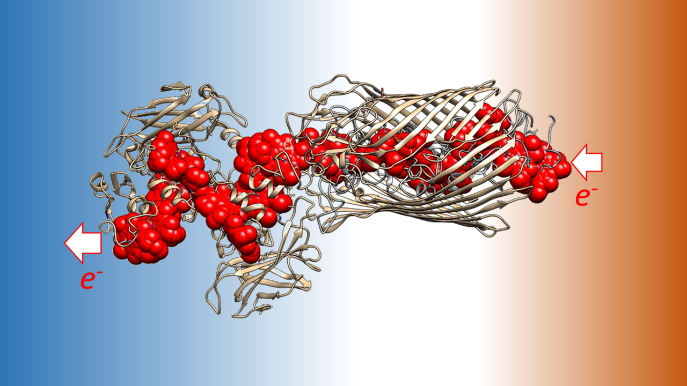
UV/visible spectroscopy and protein film electrochemistry measure the rates at which electrons move through the cytochromes. In protein film electrochemistry this movement is quantified as a flow of electrical current when the cytochrome exchanges electrons with a graphite electrode onto which it is adsorbed.
UV/visible spectroscopy detects the change of colour that accompanies the Fe3+ to Fe2+ transition of hemes. Electron transfer is triggered by illuminating dyes which absorb visible light and become strong reducing agents that pass photoenergized electrons to the cytochromes.
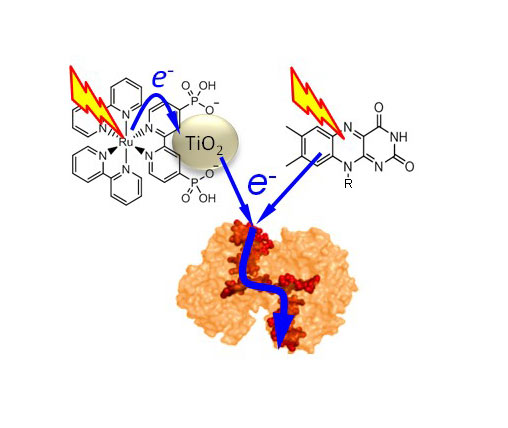
By understanding how electrons move through multiheme cytochromes UEA scientists hope to inspire the design of bespoke proteins to improve microbial fuel cells for sustainable electricity production. In addition they anticipate their work will allow the rational development of proteins with tailored properties for diverse applications within bioelectronics.
Research by Professor Butt and Dr Clarke is funded by the BBSRC and EPSRC and performed in collaboration with colleagues at UEA in addition to the University of Leeds, University of Cambridge and University College London.
References
Ultrafast Light-Driven Electron Transfer in a Ru(II)tris(bipyridine)-Labeled Multiheme Cytochrome
https://pubs.acs.org/doi/10.1021/jacs.9b06858
Structural modelling of an outer membrane electron conduit from a metal-reducing bacterium suggests electron transfer via periplasmic redox partners.
https://www.jbc.org/content/293/21/8103.full
Photoreduction of Shewanella oneidensis Extracellular Cytochromes by Organic Chromophores and Dye-Sensitized TiO₂
www.onlinelibrary.wiley.com/doi/10.1002/cbic.201600339/full
A decahaem cytochrome as an electron conduit in protein-enzyme redox processes.
pubs.rsc.org/en/content/articlelanding/2016/cc/c6cc02721k#!divAbstract
Redox linked flavin sites in extracellular decaheme proteins involved in microbe-mineral electron transfer.
www.nature.com/articles/srep11677
A decaheme cytochrome as a molecular electron conduit in dye-sensitized photoanodes.
www.onlinelibrary.wiley.com/doi/10.1002/adfm.201404541/abstract
Multi-haem cytochromes in Shewanella oneidensis MR-1: structures, functions and opportunities.
rsif.royalsocietypublishing.org/content/12/102/20141117
Case study updated January 2020